Research
Research Interests
Virus-host interactions, molecular pathogenesis of viral-associated cancers, signal transduction, extracellular vesicles, exosomes and intercellular communication.
Background
It is estimated that roughly 20 percent of all human cancers world-wide have a viral etiology or require virus infection as an essential cofactor. Epstein-Barr virus (EBV), a member of the human gamma herpesviruses, is associated with multiple human malignancies of both lymphoid and epithelial origin. EBV-associated cancers include Burkitt lymphoma, Hodgkin disease, nasopharyngeal carcinoma (NPC), and gastric carcinoma. EBV like all herpesviruses is characterized by its ability to persist for the lifetime of the host in a latent phase with sporadic reactivation and lytic replication. In EBV-associated cancers, the malignant cells contain the viral genome as an episome and express only a subset of viral genes. The latent membrane protein 1 (LMP1) of EBV is frequently detected in EBV-associated cancers and is considered the major oncogene of the virus. LMP1 is absolutely essential for EBV-induced B-cell immortalization and expression of LMP1 alone is sufficient to drive transformation of cells in vitro through the activation of cellular signaling pathways.
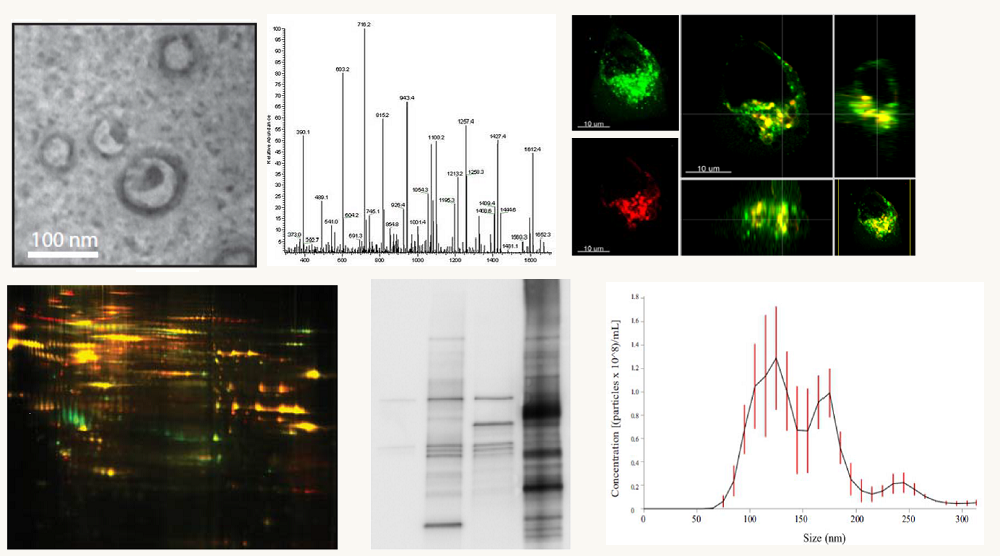
Recently, LMP1 has been found to be secreted from cancer cells in exosomes, small endocytically derived vesicles that participate in cell-cell communication. Data from our lab and others have shown that LMP1 modulates the functions and components of these vesicles. Exosomes are thought to function in many physiological processes through the transfer of biologically active proteins and RNAs between cells. Moreover, accumulating evidence suggests that alterations in normal exosome function may contribute to cancer pathogenesis. The research focus of the lab includes uncovering the mechanisms through which EBV modulates the host cell exosome pathway and determining how changes in this pathway may influence the tumor microenvironment and cancer progression. Additionally, we are interested in the molecular dissection of the cellular signal transduction pathways driving LMP1-induced transformation. To address these important questions in viral oncogenesis our laboratory employs cutting edge techniques in molecular biology, cell biology, biochemistry, and proteomics.
Research Projects
Exosome Content and Function Manipulation by Epstein-Barr Virus
Exosomes are small vesicles released at high levels from cancer cells that are important modifiers of the tumor environment and contribute to disease progression. Exosomes contain molecular information about their cells of origin and can be isolated from many biological fluids including blood, urine, and saliva. It has recently been discovered that exosomes from breast cancer cells or the blood of patients with the disease can cause non-cancerous cells to form tumors. These findings suggest a critical role of exosomes in cancer development or disease progression, and create new opportunities for exosome-based diagnostics and therapies. Based on data from our studies and others, it is also becoming clear that cancer causing viruses utilize and modify the host cell exosome pathway, and these changes may contribute to disease. For example, cells infected with the Epstein-Barr virus (EBV), a human tumor virus, release exosomes that are enriched in viral products like the major viral oncogene latent membrane protein 1 (LMP1) and virally-encoded miRNAs. We have previously shown that LMP1 alters the cargo of exosomes released from infected cells and that these LMP1-modified exosomes can exert oncogenic signaling functions on neighboring uninfected cells. In spite of the importance of inter-cellular transmission of LMP1-modified exosomes, very little is known about how this viral protein actually enters and manipulates the host exosome pathway. The overall goal of these studies is to determine the mechanisms that LMP1 drives exosome content reorganization and alters the functions of exosomes. We hypothesize that LMP1 exosomal trafficking modulates the components and biological properties of exosomes by altering endocytic routes and membrane microdomains. To test this, we aim to: 1.) investigate the mechanism through which LMP1 alters exosome components; 2.) determine the functions of LMP1-modified exosomes in intracellular communication and cellular transformation.
LMP1 Exosomal Trafficking
The overall objective of this project is to uncover the molecular basis controlling LMP1 exosome trafficking and release from the cell. We hypothesize that LMP1 traffics from the Golgi to the site of exosome formation (mutltivesicular bodies, MVBs) in Rab31/VAMP4 containing vesicles in complex with CD63. To test this, we aim to: 1.) determine the vesicular trafficking pathway traveled by LMP1 for exosome targeting using a combination of dominant negative and shRNA constructs directed against specific endocytic components and pathways identified in our preliminary studies, and 2.) investigate the LMP1-CD63 protein interaction network critical for LMP1 exosome secretion using affinity purification and quantitative mass spectrometry of interaction networks and sub-cellular compartments.
Proteomic Analysis of Cancer Exosomes for Diagnostic and Therapeutic Targets
Exosomes are released at high levels from cancer cells that are thought to modulate the tumor environment and enhance disease progression. Exosomes are stable structures that can be isolated from many biological fluids including blood, urine, and saliva. Therefore, exosomes represent a rich source of potential biomarkers to better diagnose various cancers. Our laboratory seeks to utilize exosome purification strategies that we have developed together with advanced quantitative proteomics techniques to define the protein composition of exosomes secreted from a diverse set of human cancer cell lines (the National Cancer Institute, NCI-60). The completion of this project will reveal a common set of proteins found in cancer exosomes that are likely important for their formation and function. Exosomal proteins differentially expressed in specific cancer types (e.g., breast, prostate and colon) will likely indicate disease-specific functions and reveal potential diagnostic biomarkers that will be further explored in animal models and patient samples. Overall, this project aims to understand the composition and function of exosomes secreted from cancer cells with the goal of discovering novel diagnostic targets for early detection and treatment of cancer.
Blood Exosomes and Neurodegenerative Disease
Evidence suggests that exosomes play a role in the progression of Alzheimer’s disease (AD) by transporting unwanted material between cells. The molecular information contained within exosomes may be useful in early detection of AD. Exosomes are secreted from nearly every cell type investigated; therefore, exosomes in the blood represent a complex mixture from diverse sources. For exosomes to be used routinely for diagnostic purposes it will be imperative to harvest, and enrich for exosomes originating from the brain. This study will address the current limitations of exosome-based diagnostics and provide novel strategies for molecular-based epidemiological studies. Our objectives for the study are twofold: 1) to develop techniques for identifying tissue origins of circulating exosomes, and 2) to compare and characterize brain-derived exosomes present in human blood samples from healthy, mild cognitively impaired, and AD patients. We will apply information gained from these studies to FSU College of Medicine’s geographically distributed campuses and associated large clinical network including rural and minority populations. We are well positioned to make significant advances on this area of research as our group has already developed new methods for the isolation and characterization of exosomes from blood. Overall, the proposed research will provide a novel way to detect AD risk by isolating brain-specific exosomes for early characterization. These findings will pave the way for understanding the epidemiological distribution of exosome markers in patients across the state of Florida. This study is being done in collaboration with Dr. James Olcese’s lab at FSU.
Zika Virus and Exosomes
The molecular information contained within exosomes that originates from diseased cells has proven to be useful in early diagnosis of certain cancers. Exosomes are secreted from nearly every cell type investigated; therefore, exosomes in the blood represent a complex mixture from diverse sources. Recent data suggests that exosomes from the fetus are present in the mother’s circulation and may represent a new means to non-invasively monitor the health and development of the fetus. Zika virus is an emerging infectious disease that is rapidly spreading across the Caribbean and South America with confirmed cases of locally-acquired Zika in the state of Florida. Infection of pregnant women during the first trimester has been linked to microcephaly, a neurological condition where babies are born with significantly smaller heads due to abnormal brain development. Babies born with microcephaly can develop convulsions and suffer physical and learning disabilities as they grow older. Currently, there is no non-invasive test available to determine whether a fetus has been infected with Zika virus or will develop associated disease. This study will take advantage of novel approaches to detect fetal Zika infection and to monitor the health and development of the growing fetus. Our objectives for the study are twofold: 1) to compare and characterize fetal-derived exosomes present in blood of healthy and Zika infected pregnant women; and 2) to compare molecular information in exosomes to fetal imaging data acquired during pregnancy. We are well positioned to make significant advances on this area of research as our group has already developed new methods for the isolation and characterization of exosomes from blood, including brain exosomes. Overall, the proposed research will provide a novel way to detect microcephaly risk due to Zika infection by isolating fetal-specific exosomes for early characterization from pregnant women.
Research Support
.png)

