Gallery
Research Images
Smiling face from an EM image of a Drosophila
Kc167 cell.
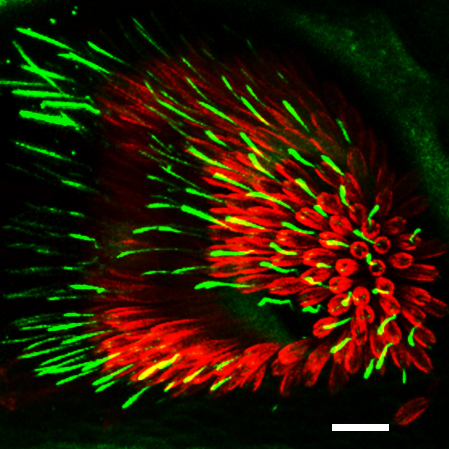
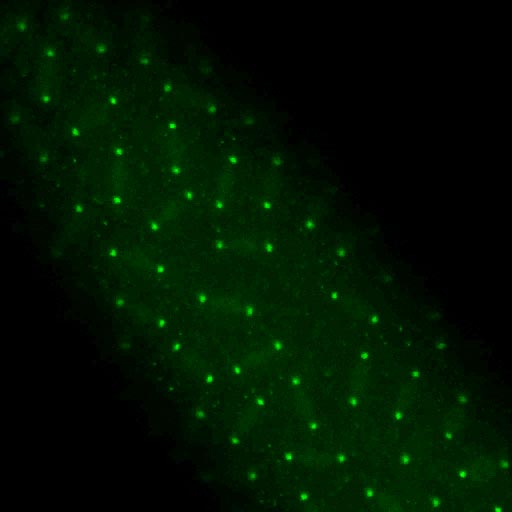
Rootletin (green) localizes to prominant ciliary
rootlets in chordotonal neurons in the Johnston's
organ, the insect auditory organ.
The scolopale rod that surrounds the cilia is labled
with Actin (red). Scale bar, 10 μm.
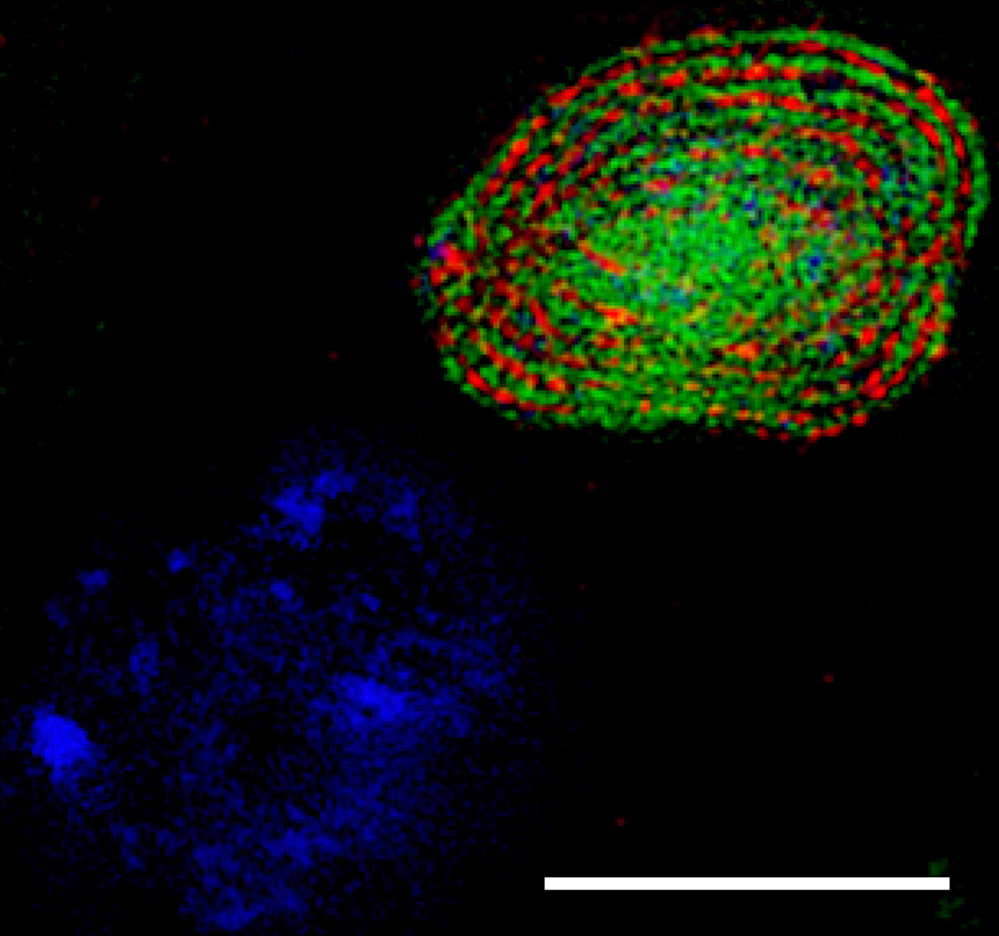
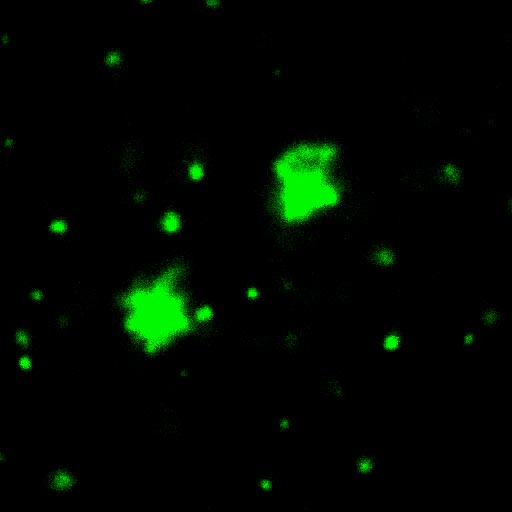
Superresolution microscopy (3D-SIM) image
showing that Spermitin (red) localizes to the matrix
of the giant mitochondria in a Drosophila
onion-stage spermatid. Mitochondrial inner
membrane is labled by ATP synthase (green) and
DNA is marked in blue. Scale bar, 10 μm.
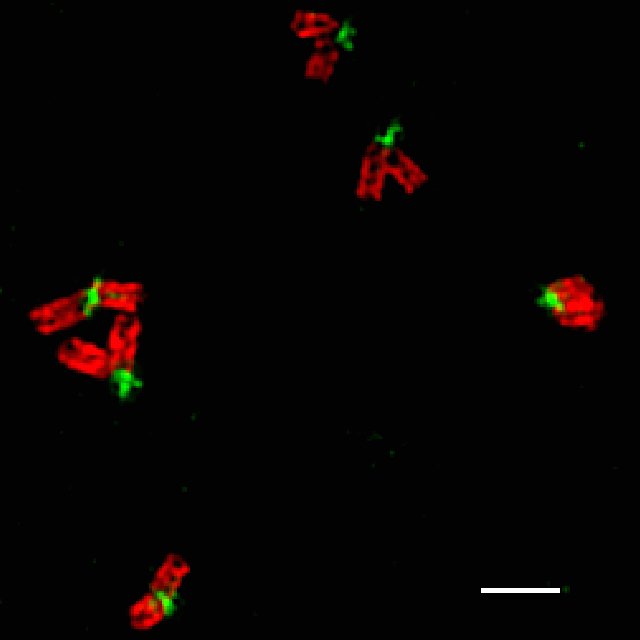
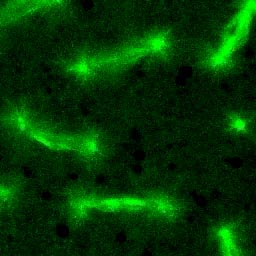
Superresolution microscopy (3D-SIM) image
showing big "V"-shaped centriole pairs in Drosophila
spermatocytes. γ-Tubulin (red) marks the centiole wall
and GFP-Rootletin (green) resides at the base of
the mother centriole. Scale bar, 500nm.
Journal Cover Art

Videos
Movies of Drosophila embryos expressing GFP-fusions to Centrosomin, Tubulin, Histone, and injected with a variety of dyes and drugs and examined in wild-type and cnn mutants.
GFP-Cnn expression in the early Drosophila embryo. Shown is nearly the entire embryo through two complete cleavage cycles.
 GFP-Cnn embryos injected with rhodamine-labeled tubulin. The dynamics and centrosomin and the microtubules can be followed simultaneously. Particles, or "flares", are seen to emerge from the centrosomes in this movie.
GFP-Cnn embryos injected with rhodamine-labeled tubulin. The dynamics and centrosomin and the microtubules can be followed simultaneously. Particles, or "flares", are seen to emerge from the centrosomes in this movie.
 Close-up view of GFP-Cnn centrosomes to show the highly dynamic nature of the flares, and how the flaring activity changes with the cleavage cycle (highest at interphase and lowest at metaphase).
Close-up view of GFP-Cnn centrosomes to show the highly dynamic nature of the flares, and how the flaring activity changes with the cleavage cycle (highest at interphase and lowest at metaphase).
Flare movement is dependent upon microtubles. Injection of GFP-Cnn embryos with colchicine, a microtubule-destabilizing drug, arrests flare particle movement.
GFP-Tubulin (Movies 5A and 5B) and GFP-Cnn (Movies 5C and 5D) embryos treated with the microtubule-stabilizing drug paclitaxel (Taxol) (Movies 5B and 5D) and control treatment (Movies 5A and 5C). Stabilizing microtubules reduced flare activity drastically, indicating that flare movement depends more upon inherent microtubule dynamics rather than being transported by microtubule-based motor proteins. Go here to see all the movies (5A-D).
GFP-Cnn embryos injected with rhodamine-labeled actin. Flare particles are seen to be restricted in their movement by the pseudocleavage furrow. The flare particles transit right up to the edge of the actin cage.
GFP-Cnn embryos injected with cytochalasin-D to inhibit actin microfilament polymerization. Actin is not required for flare movement, and flares more readily jump from one centrosome to another.
See movie of primary culture of Drosophila muscle cells prepared by Alain Debec (http://med.fsu.edu/sites/default/files/userfiles/Image/Megraw%20Lab/cultureprimairemuscles.mov)