Proposal Submission Process and Timeline
Proposal Submission Process
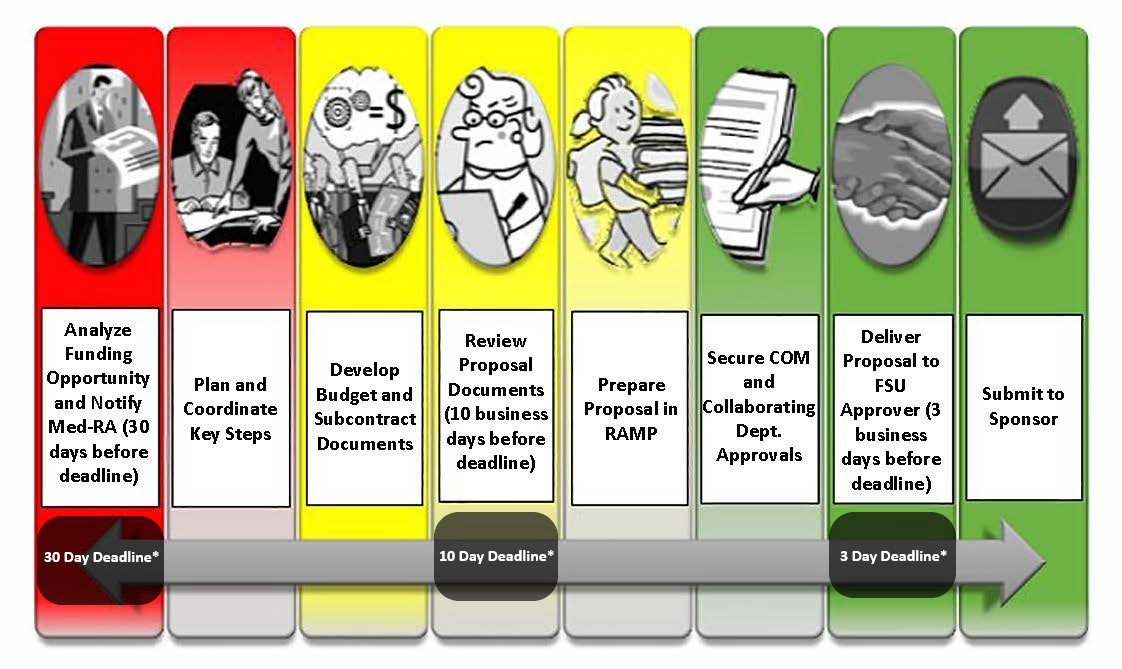
The diagram above indicates a logical and simple process flow, but the journey from finding a funding opportunity to proposal submission can be challenging. Our goal is to help you navigate that journey and minimize administrative burden, allowing you to focus on your project planning and proposal content development. All funding proposals must be approved by College of Medicine leadership and by the institution before delivery to the sponsor, so there are many rules, policies and systems applied to the submission process – your Med-RA Grants Administrator can manage all of that for you. Reach out to us at research@med.fsu.edu as soon as you identify a potential funding opportunity and we'll assign a Grants Administrator to help you get started!
Proposal Submission Deadlines
The most important component of a smooth submission is adequate time for proposal preparation and approvals. College and institutional deadline policies exist to ensure agreement from all parties to the proposal, ensure compliance with established institutional and sponsor policies, and ensure time to address potential technical submittal system issues or delays.
Help us help you! The sooner you let us know of your intent to submit a funding proposal, the sooner we can help you plan a realistic timeline for your successful submission. At least 30 days notice gives Med-RA time to confirm eligibility, facilitate subcontract development, and make sure adequate support resources are available for your submission. 60 days notice is preferred for P series and other complex grant programs. The Med-RA 10 day proposal review deadline allows us to identify and resolve potential approval roadblocks. The institutional 3 day review deadline allows FSU Sponsored Research Administration (SRA) and FSU Research Foundation (FSURF) approvers time to resolve compliance issues and address submittal system complications.
Administrative planning for proposals to the military, or those including multiple subcontracts, multiple on-campus collaborations, foreign components, mandatory cost-sharing or other complex requirements should begin as soon as possible, or within 3 months of the sponsor's due date.