Research
Interested in volunteering for research? Fill out this survey to be considered for current and future studies: Go to Survey
Or contact us at (850) 644-2824 / FSUN@med.fsu.edu
Actively Recruiting
Virtual Reality Mindfulness and Accelerated TMS Treatment for Individuals with PTSD
Purpose: Treatment study aiming to use technology to improve the treatment of PTSD.
- Age Range: 18-65
- Condition: Individuals with PTSD
- Treatment Protocol:
- Virtual Reality Guided Mindfulness: once per day for 10 days (two weeks).
- (If symptoms persist) Accelerated transcranial magnetic stimulation (TMS) treatment Week 1: 5 sessions per day for 5 days. Participants are randomized into active or sham TMS.
- Accelerated TMS Week 2: 5 sessions per day for 5 days. All participants will receive active TMS treatment during this week.
- Three follow-up visits at 1-, 3-, and 6-months post treatment.
- Some compensation provided for evaluation visits.
Neuromodulation for Postpartum Depression
Purpose: Treatment study aiming to improve symptoms related to Postpartum Depression.
- Age range: 18+
- Condition: Individuals with Postpartum Depression
- Treatment Protocol:
- Virtual Reality Guided Mindfulness: once per day for 10 days (two weeks). Five weekly follow-up sessions if you experience a benefit from treatment.
- (If symptoms persist) Accelerated transcranial magnetic stimulation (TMS) treatment Week 1: 5 sessions per day for 5 days. Five weekly follow-up sessions if you experience a benefit from treatment.
- (If symptoms persist) Accelerated TMS Week 2: 5 sessions per day for 5 days. Five weekly follow-up sessions.
Personalized TMS Dosing for Individuals with PTSD (NON-TREATMENT)
Purpose: Technology development study aiming to improve the treatment of PTSD.
- Age Range: 18-70
- Condition: Individuals with PTSD
- Protocol:
- This study uses Transcranial Magnetic Stimulation (TMS) and a measure of brain activity called functional Near-Infrared Spectroscopy (fNIRS).
- Three study visits:
- Initial office visit for evaluation and TMS motor threshold
- MRI scan of your brain
- Simultaneous TMS and fNIRS testing session
- This is NOT a treatment study, so compensation will be provided for your time.
Quality Improvement Survey: Community Perception of Neuromodulation Research
We created a quality improvement survey available to all members of the greater Tallahassee community to give us feedback on a variety of aspects of the type of research that we do. Our primary goal is to identify and engage community partners, particularly those representing groups with unmet mental health needs. We would greatly appreciate if you took a few minutes of your time to fill out our survey! Available at this link: click here. You may also scan the QR code with your device. All responses are anonymous and no identifying information will be gathered.

Not Currently Recruiting
Neuromodulation for Depression, Anxiety, PTSD and Pain
Purpose: Treatment study aiming to improve symptoms related to Anxiety, Depression, PTSD and Chronic Pain.
- Age range: 18+
- Condition: Individuals with Depression, Anxiety, PTSD, and/or Chronic Pain
- Treatment Protocol:
- Virtual Reality Guided Mindfulness: once per day for 10 days (two weeks). Five weekly follow-up sessions if you experience a benefit from treatment.
- (If symptoms persist) Accelerated transcranial magnetic stimulation (TMS) treatment Week 1: 5 sessions per day for 5 days. Five weekly follow-up sessions if you experience a benefit from treatment.
- (If symptoms persist) Accelerated TMS Week 2: 5 sessions per day for 5 days. Five weekly follow-up sessions.
MagVenture MagPro R30 TMS Machine
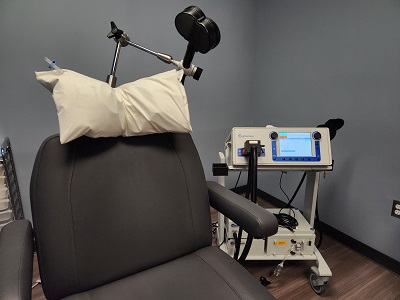
Coils: C-B70, Cool-B70, Cool-B70 A/P and Cool D-B80 A/P
More info can be found at MagVenture's website.
Magstim Rapid2 TMS Machine

Coils: Active/Placebo Rapid Airfilm
More info can be found at MagStim's website.
NeuroStar TMS Machine
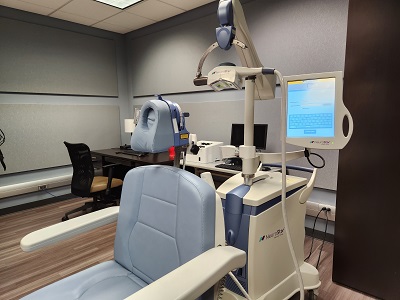
Coils: Active/Placebo iron core
More info can be found at NeuroStar's website.
Brainsight® Neuronavigation with Specialized TMS Chair
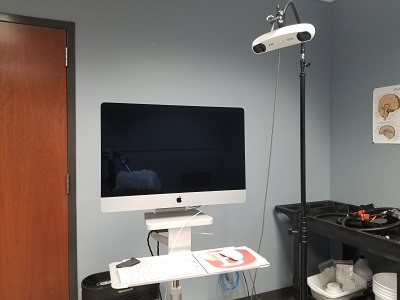
This is a Neuronavigation system with many features that allows us to target specific areas of the brain using a participant's MRI scan for TMS research. It also has tools that allow us to reconstruct brain images, including dermal layers.
More info can be found at Brainsight's website.
NIRx NIRScout fNIRS System

We use a 12-channel system for measuring oxy/deoxyhemoglobin levels in the brain during TMS sessions for research. This helps us understand what parts of the brain are activated during stimulation.
More info can be found at NIRx's website.
Compumedics Neuroscan EEG System

We use 64-channel Quik-Caps for EEG scans in our research studies. EEG (electroencephalography) allows us to measure electrical activity in the brain when a stimulus is presented (i.e., sound, images).
More info can be found at the Compumedics website.
Valve Index VR System and Oculus Quest 2 VR System


These VR (virtual reality) systems give the user an immersive virtual experience. Both are made to be comfortable for all head shapes and sizes. We currently use VR for mindfulness research protocols.
More info can be found at the Valve and Oculus websites.
Computers/Software
Lenovo ThinkPad X390 Yoga laptops and monitors. 27” iMac for the Brainsight Neuronavigation system. Microsoft Office, Adobe, MATLAB, SPSS, SAS, PsychoPy, Steam, and REDCap software.
Innovative Treatment Trial for Veterans with PTSD
The purpose of this study is to learn more about Post-Traumatic Stress Disorder (PTSD) and to test whether repetitive Transcranial Magnetic Stimulation (rTMS) alone, Cognitive Processing Therapy (CPT) alone, or rTMS combined with CPT can improve clinical outcomes. We are looking for veterans between the age of 18-60 years who suffer from PTSD. Potential participants will be screened by phone for eligibility. We expect volunteers will be in this research study for about 15 months. About 12 weeks will spent in the treatment portion of the study, and the remaining time will be for periodically monitoring. Volunteers will need to come for 18 planned study visits. Some study visits may be longer or shorter depending on how quickly the clinical evaluations are completed.
Characterizing Scalp Tolerability of TMS (NON-TREATMENT)
The purpose of this non-treatment study is to get a better understanding of the difference in how Transcranial Magnetic Stimulation (TMS) feels on different parts of the scalp. This study is for those aged 18 and above with an interest in participating in a non-treatment study. There is one study visit in which participants will undergo a brief safety evaluation and complete rating scales, and be stimulated on various parts of the scalp with the TMS coil. Compensation will be provided for your time.

